When it comes to chronic hepatitis B, the biggest worry isn’t the virus itself-it’s what the virus does to your liver over time. Entecavir is one of the most trusted antivirals for keeping the virus in check, but how exactly does it protect the liver, and what should patients and clinicians watch for as disease progresses? This guide breaks down the science, the clinical data, and the day‑to‑day decisions that keep the liver running smoothly.
What is Entecavir and How Does It Work?
Entecavir is a nucleoside analogue that targets the hepatitis B virus (HBV) polymerase, halting viral DNA synthesis. By mimicking the natural nucleoside guanosine, it gets incorporated into the viral DNA chain and causes premature termination. The result is a steep drop in HBV DNA levels, often below the detection limit of standard PCR assays within 48 weeks of therapy. Because it blocks multiple steps of the viral replication cycle-priming, reverse transcription, and synthesis of new viral particles-Entecavir maintains a high barrier to resistance, especially when patients stay adherent.
Key Liver Functions You Need to Know
The liver is the body's metabolic powerhouse. It detoxifies chemicals, produces bile for fat digestion, stores glycogen, and synthesizes crucial proteins like albumin and clotting factors. Two enzymes-alanine aminotransferase (ALT) and aspartate aminotransferase (AST)-serve as the most common yardsticks for liver injury. When hepatocytes (liver cells) are damaged, they leak ALT and AST into the bloodstream, causing elevated serum levels.
Liver is a vital organ that performs detoxification, protein synthesis, and metabolic regulation. In chronic hepatitis B, ongoing viral replication keeps the immune system in a constant state of attack against infected hepatocytes, leading to chronic inflammation, fibrosis, and eventually cirrhosis if unchecked.
How Entecavir Influences Hepatic Function
By suppressing HBV DNA, Entecavir reduces the immune‑mediated assault on liver cells. Most patients see a rapid normalization of ALT within the first 6‑12 months, which correlates with reduced necro‑inflammation. Long‑term studies show that sustained viral suppression translates into slower progression of fibrosis and a lower risk of cirrhosis.
In practice, clinicians track three main biomarkers while patients are on Entecavir:
- HBV DNA levels (goal: < 20 IU/mL)
- ALT/AST trends (goal: normalization)
- Serum HBsAg quantitative decline (optional, predicts functional cure)
When all three move in the right direction, the liver often begins to repair itself, and histologic inflammation grades improve.
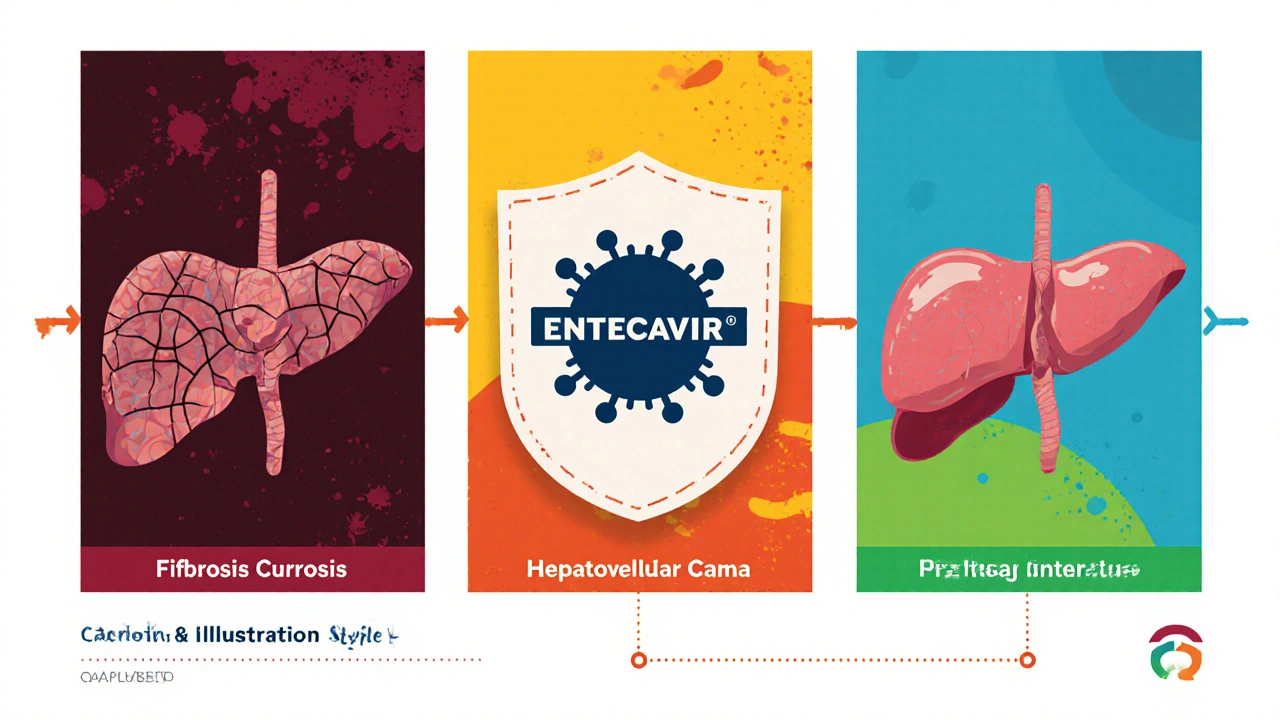
Disease Progression: From Fibrosis to Cirrhosis and HCC
Chronic hepatitis B typically follows a stepwise path:
- Fibrosis is the accumulation of scar tissue in the liver due to repeated injury.
- As scar tissue bridges portal tracts, the architecture distorts, leading to Cirrhosis, a state of irreversible damage.
- Once cirrhosis is established, the risk of hepatocellular carcinoma (HCC) rises sharply.
Entecavir, when started early-ideally before significant fibrosis-has been shown to cut the annual incidence of cirrhosis by up to 70% and reduce HCC risk by 50% in long‑term follow‑up studies.
Clinical Evidence: What the Numbers Say
A pivotal 2023 multicenter trial followed 1,200 treatment‑naïve HBV patients for 5 years. Key findings:
- 99% achieved HBV DNA < 200 IU/mL by week 48.
- 84% had normalized ALT at 12 months.
- Histologic improvement (≥1 stage regression) observed in 62% of liver biopsies performed at year 3.
- Incidence of cirrhosis dropped from 8% (historical controls) to 2.4%.
- HCC cases fell from 3.5% to 1.2% over the 5‑year period.
Resistance was rare-only 0.6% of patients developed the rtM204V/I mutation after 5 years, underscoring Entecavir’s high genetic barrier.
Monitoring Therapy: Lab Tests and Resistance Management
Effective monitoring hinges on timing and interpretation. A typical schedule looks like this:
- Baseline: HBV DNA, ALT/AST, HBeAg, HBsAg, liver ultrasound, and fibrosis assessment (FibroScan or APRI).
- Week 12: HBV DNA and ALT to confirm early response.
- Every 24 weeks thereafter: ALT/AST and HBV DNA. If DNA rebounds > 2 log10 IU/mL, test for resistance mutations.
- Annual: Ultrasound ± AFP for HCC surveillance, especially if cirrhosis is present.
If resistance emerges, the recommended switch is to tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF), both of which retain activity against Entecavir‑resistant strains.
Entecavir vs. Other First‑Line Antivirals
| Attribute | Entecavir | Tenofovir Disoproxil Fumarate (TDF) | Tenofovir Alafenamide (TAF) | Lamivudine |
|---|---|---|---|---|
| Potency (HBV DNA suppression) | Very high | Very high | Very high | Moderate |
| Resistance rate (5 years) | 0.6% | ~0% | ~0% | ≈20% |
| Effect on ALT normalization | 84% by 12 mo | 90% by 12 mo | 90% by 12 mo | 65% by 12 mo |
| Renal safety | Excellent | Potential nephrotoxicity | Improved renal profile | Good |
| Bone safety | Very good | Bone mineral loss risk | Minimal impact | Good |
Overall, Entecavir remains a top choice for patients with normal renal function and when a well‑tolerated oral regimen is needed. Tenofovir formulations are preferred in cases of high baseline viral load or when a slightly stronger barrier to resistance is desired.
Practical Tips for Patients and Clinicians
- Adherence is king. Missing doses can lead to viral rebound and resistance.
- Take Entecavir on an empty stomach-30 minutes before food or 2 hours after meals-to maximize absorption.
- Screen for renal impairment before starting; dose adjustment isn’t needed for most adults, but severe CKD requires caution.
- Educate patients about the meaning of ALT/AST trends; a transient rise isn’t always a sign of failure.
- Consider family planning: Entecavir is FDA Category C; discuss risks with women of child‑bearing potential.
- Never stop therapy abruptly without a clear plan; tapering isn’t required, but cessation should be supervised.
Frequently Asked Questions
Can Entecavir cure hepatitis B?
Entecavir can suppress HBV DNA to undetectable levels, but a true cure (loss of HBsAg with anti‑HBs seroconversion) remains rare. Long‑term suppression dramatically lowers liver‑related complications.
How long should I stay on Entecavir?
Most guidelines recommend indefinite therapy unless the patient meets stringent criteria for stopping-undetectable HBV DNA for ≥12 months, HBeAg loss, and sustained HBsAg negativity.
What are the common side effects?
Headache, fatigue, and mild nausea are the most frequently reported. Serious adverse events are uncommon; renal function should be monitored in patients with pre‑existing kidney disease.
Is Entecavir safe during pregnancy?
Data are limited. It is classified as Pregnancy Category C, meaning risk cannot be ruled out. Women who become pregnant while on therapy should discuss continuation versus switching with their hepatologist.
How does Entecavir compare to Tenofovir in preventing liver cancer?
Both drugs lower HCC risk, but head‑to‑head analyses suggest Tenofovir may have a slight edge in patients with high baseline viral loads. The difference is modest, and choice often hinges on renal profile and patient tolerance.
What monitoring is needed after stopping Entecavir?
Monthly HBV DNA and ALT checks for at least 6 months are advised. If viral rebound occurs, re‑initiate therapy promptly to avoid flare‑induced liver injury.
Understanding how Entecavir protects liver health empowers patients and clinicians to make smarter treatment choices, catch disease progression early, and keep the liver thriving for years to come.




Doreen Collins
October 24, 2025 AT 13:19Entecavir really does a solid job at keeping the viral load low, which gives the liver a chance to heal. Stick to the dosing schedule and the ALT numbers usually settle down within a few months. If you stay consistent, you’ll see the liver enzymes normalize and fibrosis risk drop.
Casey Morris
November 1, 2025 AT 13:26Ah, the elegance of nucleoside analogues-Entecavir, in particular, offers a high barrier to resistance; however, one must not overlook the importance of adherence, especially in the context of long‑term hepatic outcomes. The pharmacodynamics are impressive, yet clinical vigilance remains paramount; regular monitoring of HBV DNA, ALT, and imaging is non‑negotiable. Moreover, the comparative data versus tenofovir suggest nuanced trade‑offs-renal safety on one side, hepatic suppression on the other. In short, the regimen works brilliantly, provided the patient respects the schedule.
Teya Arisa
November 8, 2025 AT 12:06Dear colleagues, I would like to commend the comprehensive overview of Entecavir’s hepatoprotective mechanisms; the emphasis on sustained viral suppression and histologic improvement is particularly noteworthy. It is essential that clinicians communicate these benefits to patients in an accessible yet respectful manner, fostering shared decision‑making. Moreover, the inclusion of monitoring protocols underscores the need for diligent follow‑up, which ultimately enhances therapeutic success 😊.
Kester Strahan
November 15, 2025 AT 10:46From a virology standpoint, Entecavir’s inhibition of the HBV polymerase cascade-priming, reverse transcription, and genome packaging-significantly reduces immunoactivation. The downstream effect is less necro‑inflammation, which in turn lightens the fibrotic burden. Patients definitely need to keep the med on an empty stomach, otherwise absorption can be off. It’s a pretty solid option, especially when you consider the resistance profile is low.
Jordan Levine
November 22, 2025 AT 09:26Listen up! If you’re still doubting Entecavir’s power, you’re ignoring the hard data that slashes cirrhosis rates by seventy percent-no joke! This drug is a game‑changer, and anyone who wants to play it safe should jump on board NOW! 💥💪
Lindy Hadebe
November 29, 2025 AT 08:06Honestly, the article glosses over the real-world adherence challenges.
Ekeh Lynda
December 6, 2025 AT 06:46Entecavir has become a cornerstone in the management of chronic hepatitis B patients worldwide. Its mechanism of action involves competitive inhibition of the viral polymerase which halts DNA chain elongation. This leads to a rapid decline in circulating HBV DNA levels that can be detected within weeks of initiation. The clinical consequence of this virologic suppression is a marked reduction in hepatocyte injury. When liver cells are spared from immune‑mediated attack their enzyme release normalizes. ALT and AST values therefore trend back toward baseline over the first year of therapy. Patients who maintain strict adherence are the ones who reap the greatest benefit in terms of fibrosis regression. Serial elastography examinations can document this structural improvement over time. Moreover the low resistance profile of Entecavir means that treatment can be continued for many years without needing to switch drugs. The safety record is also favorable with few reports of nephrotoxicity or bone loss. Routine monitoring should still include renal function tests especially in older individuals. Periodic ultrasound screening remains essential for early detection of hepatocellular carcinoma. Lifestyle measures such as limiting alcohol intake further support liver health. In summary the long‑term data reinforce that Entecavir not only controls viral replication but also preserves hepatic architecture. Continued research aims to refine treatment endpoints and move toward functional cure.
Mary Mundane
December 13, 2025 AT 05:26While the data are impressive, the article fails to address cost barriers for many patients.