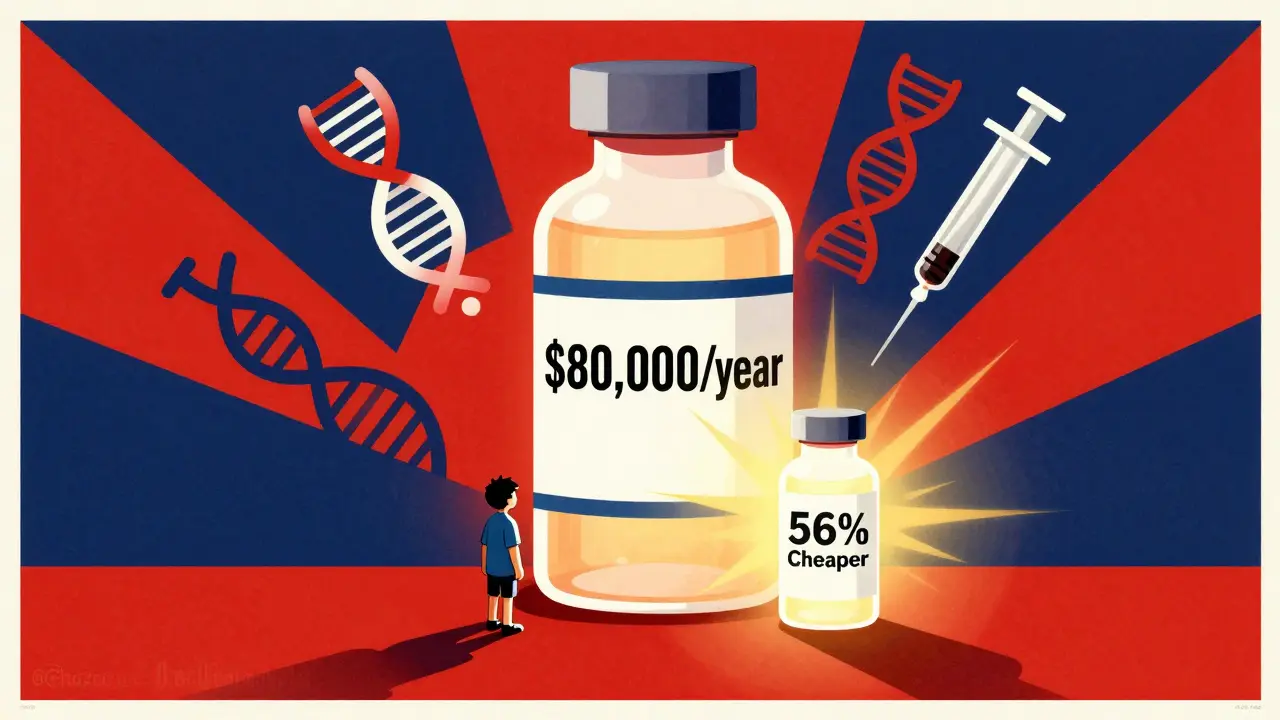
When you’re prescribed a biologic drug - say, for rheumatoid arthritis, Crohn’s disease, or cancer - you might see a price tag of over $80,000 a year. That’s not a typo. Now imagine a version of that same drug costing less than half, with the same safety and effectiveness. That’s not science fiction. It’s a biosimilar. And the cost difference between brand biologics and their biosimilar versions isn’t just noticeable - it’s life-changing.
What Exactly Is a Biosimilar?
People often confuse biosimilars with regular generics. They’re not the same. Regular generics are copies of simple, chemically-made drugs. Think ibuprofen or metformin. You can recreate them exactly in a lab. Biosimilars are different. They’re copies of biologic drugs, which are made from living cells - like proteins, antibodies, or enzymes. These are huge, complex molecules. Even tiny changes in how they’re made can affect how they work. So, a biosimilar isn’t an exact copy. It’s highly similar. And that’s enough. The FDA requires biosimilars to prove they work just like the original biologic in clinical trials. No guesswork. No shortcuts. They must match in safety, purity, and potency. In fact, the FDA says approved biosimilars are as safe and effective as the brand-name versions. And yet, they cost far less.How Much Do They Actually Save?
Let’s look at real numbers from 2025. The average 30-day cost of a brand biologic in the U.S. was $2,104. The biosimilar version? $919. That’s a 56% drop. For a patient on monthly treatment, that’s over $14,000 saved per year. For someone on long-term therapy - say, 10 years - that’s more than $140,000 in savings. Take Humira, the world’s best-selling drug for years. Before biosimilars hit the market, a single year’s supply cost about $80,000 in the U.S. After biosimilars launched in 2023, the price dropped by 80%. Sandoz’s Hyrimoz, one of the first biosimilars, captured 14% of the market in just six months. Today, biosimilars hold about 65% of the adalimumab market. That’s not just competition - it’s a market reset. And it’s not just Humira. Biosimilars for drugs like Enbrel, Remicade, and Herceptin have followed the same pattern. On average, biosimilars launch at 50% off the brand price. Over time, that discount grows. Brand companies often lower their own prices to stay competitive - sometimes by 25% or more. That means savings pile up for everyone: patients, insurers, hospitals.Why Aren’t More People Using Them?
If biosimilars are cheaper and just as good, why aren’t they everywhere? The answer isn’t science. It’s money. Big pharmaceutical companies don’t want to lose their billion-dollar profits. So they’ve built legal and financial walls around their drugs. One trick? Patent thickets. That’s when a company files dozens - sometimes hundreds - of minor patents on tiny changes in packaging, delivery methods, or dosing schedules. Each one delays a biosimilar’s entry. Some of these patents have nothing to do with the actual medicine. They’re just delays. Then there are Pharmacy Benefit Managers (PBMs). These are the middlemen between drug makers and insurers. They get rebates - big kickbacks - from brand biologic makers. The higher the drug’s price, the bigger the rebate. So even if a biosimilar is cheaper, the PBM might push the brand drug because it pays them more. That’s not a mistake. It’s the business model. Doctors and pharmacists often don’t know the difference between biosimilars and brands. Some still think biosimilars are “inferior.” That’s outdated. The FDA has approved 76 biosimilars as of October 2025. None have been pulled for safety issues. But awareness is still low.
Who’s Saving the Most?
Patients aren’t the only ones saving. The whole system is. Since 2015, biosimilars have saved the U.S. healthcare system between $36 billion and $56 billion, depending on who’s counting. In 2024 alone, $20 billion was saved. That’s enough to cover free insulin for millions of Americans. But here’s the kicker: biosimilars make up less than 20% of the biologic market. Compare that to traditional generics - which cover 90% of prescriptions but only 13% of total drug spending. Why? Because generics are simple. They’re cheap to make. Biosimilars cost $100-250 million to develop. That’s why fewer companies make them. Still, the numbers are turning. Evaluate Pharma predicts biosimilar market share will hit 35-40% by 2030. That could mean $125 billion in annual savings. If we fix the rebate system, streamline approvals, and ban patent abuse, we could save another $42.9 billion by 2027, according to HHS.What’s Changing in 2026?
The FDA just released new draft guidance to cut red tape in biosimilar development. Less clinical testing. Faster approval. Lower costs to make them. That’s huge. The Biden administration’s Biosimilars Action Plan is pushing for better reimbursement rules and transparency in PBM rebates. Some states are now requiring pharmacists to substitute biosimilars unless the doctor says no. Insurers are starting to notice too. More are putting biosimilars on the lowest cost tier. Some are even refusing to cover the brand drug unless the patient tries the biosimilar first. That’s called step therapy - and it’s working. The real game-changer? New biosimilars for the biggest sellers are coming. By 2027, biosimilars for Ocrevus, Stelara, and Keytruda will hit the market. Those drugs each bring in over $10 billion a year. When biosimilars arrive, prices will crater. Again.
What Should You Do?
If you’re on a brand biologic:- Ask your doctor if a biosimilar is an option.
- Call your pharmacy - they might have one in stock.
- Check your insurance formulary. Is the biosimilar cheaper?
- If your insurer won’t cover it, ask for a prior authorization. Many will approve it if you ask.
- Share the facts. Biosimilars aren’t cheap knockoffs. They’re rigorously tested, FDA-approved, and safe.
- Push your employer or union to switch to biosimilars in their health plan.
- Write to your representative. Support laws that ban patent thickets and require PBM transparency.
The Bigger Picture
Biologics are the future of medicine. They treat diseases we couldn’t touch 20 years ago. But they’re also the reason drug prices keep climbing. Without biosimilars, we’re stuck paying $80,000 for a drug that could cost $15,000. The science is ready. The savings are real. The only thing holding us back is a system built to protect profits - not patients. It doesn’t have to be this way. Biosimilars prove we can have cutting-edge medicine without bankrupting families. The tools are here. The data is clear. Now it’s just about making the right choices.Are biosimilars as safe as brand biologics?
Yes. The FDA requires biosimilars to undergo rigorous testing to prove they’re as safe and effective as the original biologic. They must match in structure, function, and clinical outcomes. No FDA-approved biosimilar has been withdrawn for safety reasons. Thousands of patients have used them with no increased risk.
Why are biosimilars cheaper if they’re so complex to make?
They’re cheaper because they don’t need to repeat all the early-stage research. The original biologic maker spent billions on discovery and clinical trials. Biosimilar makers use that data to prove similarity - not start from scratch. That cuts development time and cost by 60-70%. Even with $100-250 million in costs, they still price 40-80% below the brand.
Can I switch from a brand biologic to a biosimilar?
Yes, and many patients do. In fact, switching is common in countries like Germany and Canada, where biosimilars are widely used. In the U.S., doctors often switch patients after the first refill. Studies show no difference in outcomes. Always talk to your doctor first, but switching is safe and supported by major medical societies.
Why don’t my insurance plans cover biosimilars more often?
Many plans still pay rebates to brand drug makers based on the drug’s list price. Since biosimilars are cheaper, they offer smaller rebates. Some PBMs (middlemen) push the more expensive brand drug because it pays them more. That’s changing as more insurers adopt step therapy and require biosimilar use unless there’s a medical reason not to.
Will biosimilars eventually replace brand biologics?
They won’t replace them - they’ll compete with them. Brand biologics will still be used, especially for patients who respond better to them. But as more biosimilars enter the market, brand prices will keep falling. The goal isn’t to eliminate brands. It’s to make treatment affordable for everyone. Right now, 80% of biologic spending goes to just 5% of prescriptions. Biosimilars fix that imbalance.





Bryan Wolfe
January 11, 2026 AT 20:45Okay, I just switched my mom from Humira to Hyrimoz last month-she’s been on it for 6 weeks now, and her joint pain? Gone. Like, actually gone. No new rashes, no weird fatigue, no ER visits. Her copay dropped from $450 to $85. I cried in the pharmacy parking lot. This isn’t theoretical-it’s real life. If your doc says ‘it’s too risky,’ ask for the FDA’s biosimilar approval docs. They’re public. And yes, I’m still mad at PBMs.
Prachi Chauhan
January 12, 2026 AT 17:05Why do we still call them ‘biosimilars’? It sounds like a compromise. Like, ‘it’s almost the same’-but it’s not. It’s the same. The molecule works the same. The immune system reacts the same. The body doesn’t know the difference. We’re stuck in a language game made by lawyers and marketers. We need a new word. ‘Copied biologic’? ‘Shared protein’? Something that doesn’t whisper ‘inferior’.
Katherine Carlock
January 12, 2026 AT 19:19I work in a rheumatology clinic. We had a patient who refused biosimilars for 2 years because her friend’s cousin ‘got sick’ on one. We showed her the FDA’s 76-approved list. Zero withdrawals. Zero safety flags. She started on a biosimilar last week. Today she sent me a selfie with her dog and a coffee. No flare. Just life. The science is solid. The fear? That’s the real drug we need to fight.
Sona Chandra
January 13, 2026 AT 03:03THIS IS A SCAM. The pharma giants are laughing all the way to the bank while patients die waiting for affordable meds. They buy politicians. They buy doctors. They buy your insurance plan. They make you feel guilty for wanting to live without bankruptcy. And now they’re calling it ‘innovation’? I’m so tired of being told to ‘trust the system’ when the system is built to bleed me dry. #StopPharmaGreed
Jennifer Phelps
January 15, 2026 AT 02:54How many biosimilars have been on the market longer than 5 years? I’ve seen the 2025 data but what about long-term outcomes? Are there studies tracking 10-year use? I’m not doubting the FDA but I need longitudinal data before I switch my treatment. Just asking.
beth cordell
January 16, 2026 AT 20:17Just got my biosimilar refill 😊 My insurance didn’t even make me jump through hoops this time! 🎉 I used to cry every time I opened the bill. Now I just say ‘thank you’ to the scientists who made this possible. Also, my cat approves. She’s been extra cuddly lately. 🐱💖
Lauren Warner
January 18, 2026 AT 07:14Let’s be real. The 56% savings? That’s on paper. Most patients still pay $300-$500 a month because insurers still require prior auths, step therapy, and formulary tiers. The real savings are going to PBMs and hospital systems-not you. And don’t get me started on how many patients get denied because their ‘specialty pharmacy’ doesn’t stock it. This isn’t progress. It’s a well-lit trap.
Craig Wright
January 19, 2026 AT 17:21As a British citizen, I find it astonishing that the U.S. still allows such grotesque pricing. In the NHS, biosimilars are standard. No negotiation. No rebates. Just cost-effective treatment. The American system is not a healthcare system-it is a market for profit extraction. This is not innovation. It is exploitation dressed in white coats. Shameful.
Lelia Battle
January 21, 2026 AT 06:27I’ve spent years thinking about this. The complexity of biologics isn’t just scientific-it’s philosophical. We treat molecules like they’re disposable commodities, but they’re woven into our biology, our identity, our survival. Yet we’re told to accept ‘similar’ as good enough. Maybe the real question isn’t whether biosimilars work-but why we’ve accepted a system that makes us feel grateful for a discount on life-saving medicine.
Cassie Widders
January 22, 2026 AT 04:39